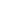- Home
- News
- Industry News
- Why Improve The Water Quality In District Heating Systems?
Why Improve The Water Quality In District Heating Systems?
- Industry News
- 15 January 2020
- by DBDH - Hot Cool Magazine
Demineralization and deaeration of district heating makeup water have for many years been a privilege for only a few major district heating companies. Lately, it has become possible for smaller and middle-sized district heating companies to benefit from new technologies to produce salt and oxygen-free district heating water.
Please note that this article was originally published in the DBDH Hot Cool magazine.
Demineralization and deaeration of district heating makeup water have for many years been a privilege for only a few major district heating companies. Lately, it has become possible for smaller and middle-sized district heating companies to benefit from new technologies to produce salt and oxygen-free district heating water.
Why Is It Important To Remove Salts And Oxygen In District Heating Make-Up Water?
Often smaller district heating (DH) companies argue that “we have always used softened make-up water. We add chemicals for alkalizing the water, scavenging oxygen and then take care of hard water that eventually breaks into our system”. Another common statement is that “when we cut our pipes we see no corrosion. If we see corrosion it must be coming from outside the pipes”. The last postulate might very well be the first impression you get when you look on a corroded pipe, but it is not always the reality.
What Causes Corrosion And Can Chemicals Avoid Corrosion?
There are four main factors promoting corrosion;
• Aggressive ions (salts) causing increased conductivity in the water
• Oxygen
• Incorrect pH
• Biological activity
Corrosion is an electrochemical reaction between oxygen, water and metal. The reaction can only happen if all three components are present. Therefore, make-up water should ideally be demineralized, deaerated and pH adjusted. Salts and oxygen serve as catalysts for corrosion. Salts increase the conductivity in water, which is a prerequisite for corrosion, and the oxygen oxidises (combines with metal ions to form rust) and corrodes the steel in the DH pipe. Due to these processes, the use of chemicals in DH systems should be reduced as much as possible because they increase the salt content and thereby the conductivity of the water circulating in the system.
Another issue with the chemicals used in DH systems is that they are often organic chemicals, which, if added to the water will serve as nutrients for bacteria growth and formation of biofilm, leading to biocorrosion below the film. They also form sludge and increase the risk of deposits, which typically occur in places where the water velocity is low, e.g. in heat and accumulation tanks. The bottoms of the tanks can suffer from severe corrosion due to deposits and sludge. Finally, it should be mentioned that the best oxygen scavenging agent in the system is the steel surface. If steel and oxygen scavenging chemicals compete for the oxygen, the steel surface always wins. If water contains oxygen the corrosion process will continue. If there is no oxygen the corrosion process will cease.
At low pH values, the corrosion process increases, and at high pH values, the risk of corrosion in copper, copper alloys and metal parts of aluminum and galvanized parts also increases. The recommended pH of DH water is 9.8 ± 0.2, and this should be achieved by dosing sodium hydroxide (NaOH). When demineralized water is used in the system, the amount of NaOH will be reduced dramatically, whereas the use of softened water calls for high use of NaOH due to its content of carbonate.
New Modern Water Treatment Technologies Without Using Chemicals
Natural drinking water is characterized by having a balanced content of cations and anions. All together these make up the total hardness, salts and gasses like oxygen (O2), carbon dioxide (CO2) and nitrogen (N2). For removal of hardness and salts the most common water treatment technologies are:
• Water softening, which removes the total hardness from water.
• Demineralization of water by use of reverse osmosis or ion exchange.
• Production of ultra-pure water where the remaining salts are removed, sometimes referred to as polishing.
As mentioned, it is very important to remove salts and oxygen because they are prerequisites for corrosion. Furthermore carbon-dioxide should be removed since it increases the conductivity and the consumption of NaOH (lye). Therefore, gasses like oxygen and carbon dioxide should be removed before the water is used as make-up water. In case oxygen enters the district heating system through leakages, the character of corrosion will change from aggressive local corrosion to general surface corrosion, which is not as critical as local corrosion. New efficient technologies have made degassing an attainable solution for all district heating companies. The recommended solution is a Membrane Degassing Unit (MDU). It should be said than an MDU can be used to degas softened water, but the superior result will be achieved using demineralized water.
Overview: Advantages Of Using Demineralized And Deaerated Make-Up Water In District Heating Systems
• The risk of corrosion is reduced because the corrosion process requires salts and oxygen to react.
• By reducing the salt content in the water, the risk of microbiological growth in the district heating system is reduced, which again reduces the risk of microbiological corrosion.
• The need for chemicals is significantly reduced (typically more than 90%) because the “buffer” in the water is reduced by reducing the salt content. The “buffer” defines how much NaOH is needed to change the pH. Water with a low buffer gets effected by NaOH more easily than water with high buffer. Therefore, less chemicals are needed for demineralized water than just softened water.
• Using demineralized water in the district heating system makes detection of raw water break-in much more efficient than in systems with just softened water. This is because raw water break-in into demineralized water creates bigger fluctuations in the parameters of the system.
• By using demineralized water with low conductivity instead of softened water, the corrosion which will occur when raw water breaks into the system will be the relatively unharmful general surface corrosion instead of aggressive local corrosion.
• An MDU-plant removes oxygen and carbon dioxide in the demineralized water by means of membrane contactors, a vacuum pump and nitrogen as a sweep gas. This method is very efficient and reduces the oxygen content in the demineralized make-up water from 5-10 mg O2/liter to below 0.02 mg O2/liter.

Case Study: Practical Experience With Improving The Water Quality In A District Heating System.
Aars District Heating Company is a CHP plant that produces and distributes electricity and heat for about 5500 households. Heat is transmitted through one transmission line to 6 distribution networks. The make-up water was previously produced in a water softener and deaerated in a traditional vacuum deaerator. In July 2018, the water treatment strategy was changed. Today the make-up water is demineralized. The make-up water is produced in a central water treatment plant, and all make-up water is added to the transmission line. All distribution networks get their make-up water from the transmission net. Since then the use for oxygen scavenging chemicals has been eliminated completely. In addition, the use for chemicals for pH adjustment has been reduced 95%.
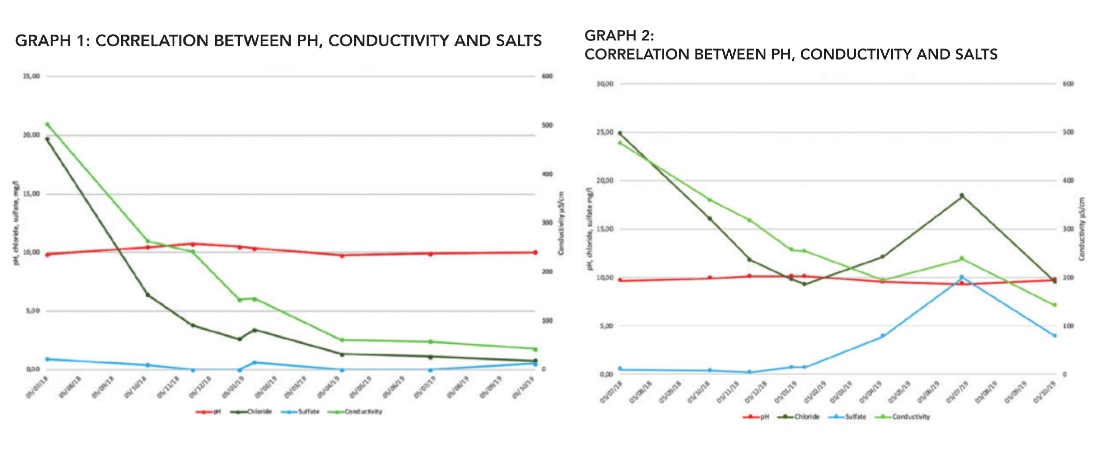
Graph 1 shows the development in the water quality in the transmission line in the period July 2018 to October 2019. In this period, the water quality has changed from softened water to demineralized water. Thus it follows the “Recommendations for Water Treatment and Corrosion Prevention”, authored by the Danish District Heating organization. In the beginning of January 2019, a system leak occurred and as an emergency, soft water with higher conductivity was used as additional make-up water.
This can be seen as the increased content of chloride, sulfate and conductivity. A slight increase in the content of sulfate can be seen October 2019. A possible explanation for this is that sulphate reducing bacteria had been reduced due to the improved water quality. The reason is that harmful bacteria simply do not get any nutrients (salts) to support life. Therefore, the bacteria do not any longer “eat” the sulphate. This explanation is supported by measurements in the content of sulphide (not shown on the graph). In the beginning of the period the level of sulphide was 0.5 mg/l but in October 2019 it was reduced to below the detection limit. Graph 2, for one of the distribution networks clearly shows problems with raw water break-in. This clearly occurs in the period from April to the beginning of July 2019, where chloride, sulphate and conductivity increases while the pH value slightly decreases. After this period the leakage stopped, and the parameters returned to normal.
Summary
Optimum water treatment will help prolong the lifetime of pipes and critical components in the DH system. By desalination, deaeration and pH adjustment, the lifetime increases. Consider a business case where the lifetime of the pipes was increased from 50 to 70 years. The important measures to avoid corrosion are:
• Reduce the conductivity in DH water by use of demineralized water and keep oxygen out of the system. Salts and oxygen are powerful catalysts for corrosion.
• Avoid unnecessary use of chemicals. Chemically speaking, oxygen reacts faster with steel than oxygen binding agents. Chemicals also increase the conductivity, the risk of forming sludge and they act as nutrients for corrosive microbiological growth.
For further information, please contact Thomas Dalsgaard – [email protected]
Latest News
-

-
 26.10.2021 Celsius Summit 2021 – Energy Democracy
26.10.2021 Celsius Summit 2021 – Energy Democracy